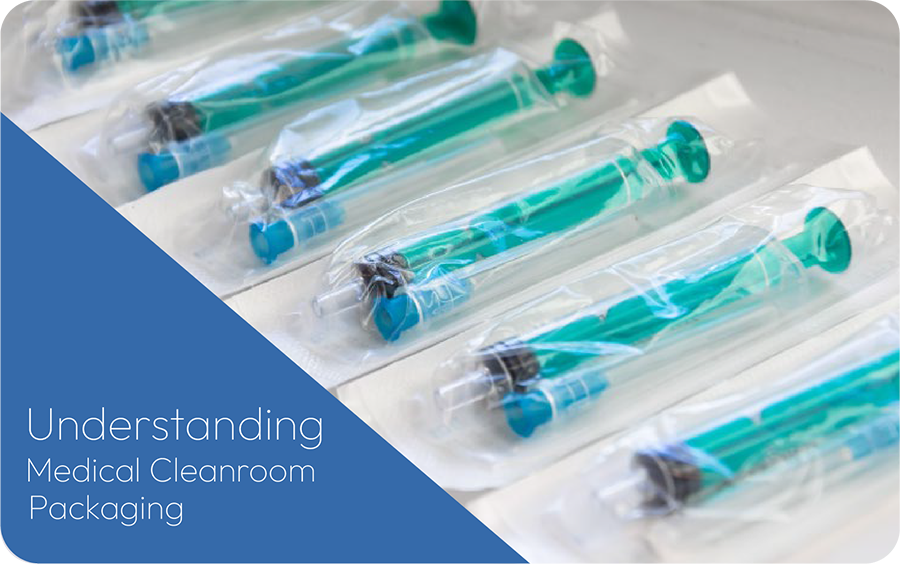
Medical research is extremely important in developing vaccines and medications, treatments, medical devices, and many other medical applications that could be used to save or improve someone’s life. Making sure these medical products are developed, produced, and packaged without being contaminated with bacteria or viruses that could potentially cause illness is crucial. Learn more about packaging for medical devices below.
Medical cleanrooms are the solution.
These rooms are required to be highly sterile and must have extra safeguards in place to protect employees and medical products from contamination. There are different classifications of medical cleanrooms depending on what is being researched and produced within them. These classifications vary in the particle size that needs to be filtered through, how often the air must be changed, and how sterile the room needs to be.
The Importance of Packaging in the Medical Device Industry
Proper packaging of medical instruments and devices ensures that the product is safe and secure during transportation and storage. Making sure the packaging properly fits the device, is made of the right materials, and has proper storage instructions prevents damage to the product and
makes sure it is in perfect condition for safe and ready use when opened.
Medical device packaging also displays important information for both the patients and the healthcare provider. Information on how to use, maintain, and store the instrument or device correctly prevents improper usage and storage, which prevents injury or contamination. Additionally, packaging normally communicates the safety risks associated with the device so the patient and healthcare worker know the risks when using the device.
Navigating Regulatory Requirements and Standards for Cleanroom Packaging
Standard and regulation requirements change for each class of cleanroom. Generally, medical device packaging takes place in an ISO Class 7 or 4 cleanroom. There are a few differences in what the requirements are for packaging medical devices in each type of cleanroom.
ISO Class 4 Cleanroom
Catering to extremely clean applications, ISO Class 4 cleanrooms are free from any kind of organic material. While it is common to use these cleanrooms for nanotechnology, however, it is also used for biotechnology or medical devices that require an extremely sterile packaging environment.
Only 10,000 0.1-µm-sized particles per cubic meter are able to enter these clean rooms. The air must be changed 300-540 times per hour, and the fan/filter unit coverage must be 50-90%. Specific ULPA filters are required to filter out all of the unwanted organic particles from entering
this extremely sterile environment.
ISO Class 7 Cleanroom
These cleanrooms have less stringent standards that must be met. ISO Class 7 cleanrooms are gaining popularity because of their clean, but efficient nature.
Class 7 cleanrooms only allow 10,000 particles to enter, the air must be changed 60-90 times an hour and the fan/filter unit must have a coverage of 15-20%. These cleanrooms meet FDA GMP standards and are widely used in the medical industry.
Impact of Regulatory Compliance on Market Access
Meeting regulatory compliance for medical device packaging is crucial to your bottom line. Failure to meet requirements and standards can result in fines, penalties, and loss of market access. It is crucial to choose a medical device packaging company that has the cleanroom specifications
necessary for packaging your product to avoid these penalties and financial losses.
Designing Cleanroom Packaging for Medical Devices
Considerations for Ensuring Safety and Sterility
When designing packaging for medical devices that need the sterility of a cleanroom, it is important to take the quality of materials into consideration. At PPC Flexible Packaging, we make sure all of our packaging products are manufactured from their own exclusive raw materials. This ensures the highest standards of quality and traceability. Our raw materials are certified to specific surface cleanliness levels.
How the packaging is sealed is also an important consideration. Sealing the packaging in certain ways prevents contamination. Your packaging manufacturer will be able to work with you to determine the best way to seal your medical device for its sterility and ease of use.
The Role of Packaging in Device Usage
Packaging serves many important roles in the life of medical devices. Not only is it what keeps the device safe and secure during transportation and storage and in perfect, ready-to-use condition when opened, but it also displays key information about how to use, store, and care for the medical device.
It is important your medical device packager is able to properly house your device to meet all criteria, as well as print helpful information on the packaging for your customers’ safety. Importance of Labeling and Instructions for Use Printing the instructions for the use, storage, and maintenance of a medical device on the packaging is important to keep healthcare providers and patients safe.
 Challenges and Solutions in Medical Device Packaging
Challenges and Solutions in Medical Device Packaging
Demand for Clean Production and Assembly is Growing
If your medical device is contaminated or damaged during storage or transit, it can harm patients. Whether it is through contamination or a faulty device, a contaminated or damaged product impacts operations and performance, causing loss of pro ts and potential nes from organizations dedicated to upholding
regulations and standards.
Working with a professional cleanroom packager can give you the resources to use the correct materials, have the proper designs, and the sterile processes through cleanrooms needed to keep your product safe and free from harmful microbes.
Meeting Cleanroom Requirements
Sometimes damage to your product is invisible. Contamination of medical devices is considered damage and will impact your product.
A professional cleanroom packaging company can provide your medical device with the proper materials and processes that will keep contaminants out of your medical device packaging. Cleanrooms that are maintained at the proper level reduce the risk of contaminants.
Meeting Customer Requirements
Every customer has a vision in mind for their product and its packaging. Making sure there is clear communication, questions are being asked, and it is clear what is needed from the product—whether that is regulation requirements, materials, or applications—can help reduce the risk of the customer getting a packaged product they do not want.
Advancements and Trends in Cleanroom Medical Packaging
Optimization of the Cleanroom Packaging Process
With all of the technological advancements we see coming to the industry, professional packaging companies are able to combine decades of technical experience with improved materials and advanced machinery to increase production rates.
Sustainable Initiatives
Eco-friendly packaging is becoming more and more important. Implementing
energy-efficient machines, and EPA-approved materials, and reassessing processes to improve efficiency and reduce wasted materials are great steps to take toward leaving a smaller carbon footprint.
Work with PPC Flexible Packaging for Your Medical Device Packaging
Our ISO Class 4 and 7 cleanrooms give us the perfect environments to package your medical devices safely in a sterile environment. All of our raw materials are certified to ensure cleanliness and traceability, so you know exactly which product you are getting and just how clean it is.
While we can’t reuse plastics for cleanroom applications, we do use Regenerative Thermal Oxidisers (RTOs) in our manufacturing plants to reduce our carbon footprint. This system destroys volatile organic compounds, such as air toxins, odors, and hazardous air pollutants, that may be created during our manufacturing processes.
If you are ready to work with an environmentally conscious manufacturer that can meet your cleanroom packaging needs, contact our team today.